
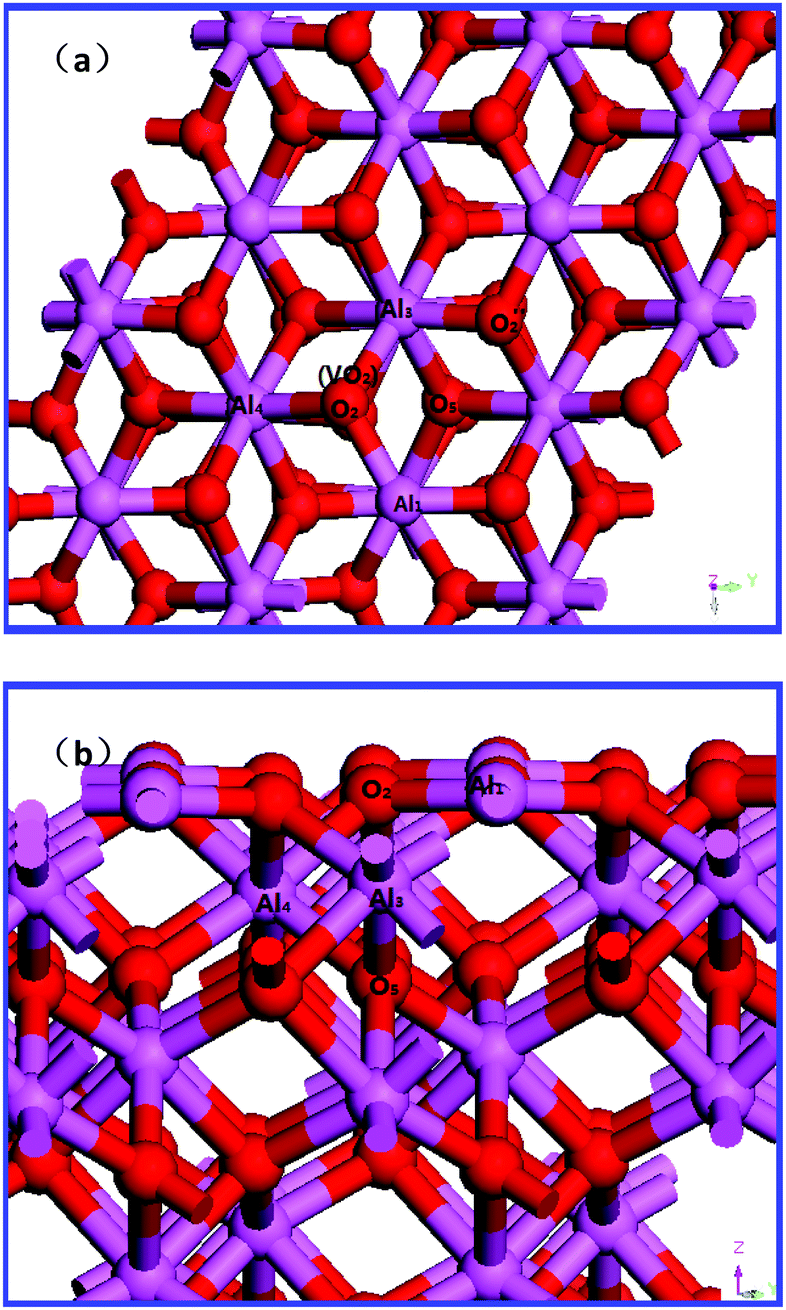
Line shows a second order polynomial fit.įigure 4 suggests that AA stacked bilayer graphene is not a local maximum, but could be transition state itself, since the RMS force at AA state was 2.5meV/A. Energy along our constructed path x_i(p) adjusted by the energy of AA configuration. The search fails at LST stage because AA stacking energy is the highest on path constructed by LST.įigure 4. The results of LST/QST search are shown on Fig 3. The reaction energy 13meV/atom was consistent with the binding energy differences obtained previously in the studies using the same method for vdW interaction.

Resultsīefore the transition state search both AA and AB stacked bilayers were geometrically optimized using the same ECUT and KPOINTS. To speed up the computations we used ECUT=500eV and KPOINTS=11x11x1, providing convergence of energy up to 0.005eV. Then QST and CG steps are repeated until the RMS force is within 0.1eV/A. The result of QST maximization is again refined by conjugate gradient minimization(CG). The obtained approximation to the transition state is then used as an intermediate state for QST. The algorithm starts with LST, followed by conjugate gradient minimization. We chose matching pattern corresponding to shifting top layer as a whole, as mentioned above. The bottom layer is trivial, but the top layer could be matched in at least two different ways. The LST/QST algorithm requires one-to-one matching of product and reactant atoms. The parameters used for optimization were ECUT=650eV and KPOINTS=17x17x1. The reactant and the product have to be geometrically optimized, which was done previously. To find transition state we employ Linear and Quadratic Synchronous Transit methods(LST/QST). To account for interlayer van der Waals(vdW) interaction we used Tkatchenko-Scheffler DFT-D correction. Since plane-wave method requires periodic boundary conditions we make a box of height h=17 Å and a cross section matching unit cell of graphene(a=2.46 Å). SCF convergence tolerance was set to 5.0E-7eV/atom. Pseudo atomic calculation is performed for 2s2 2p2 orbitals of C atoms.
We also employ On-the-fly generated (OTFG) ultrasoft pseudopotential was used to describe the interactions of ionic core and valance electrons with a core radius of 1.4Bohr(0.74 Å). With CASTEP, we use the GGA-PBE as an exchange-correlation functional. Ground state energy computations were performed using DFT plane-wave pseudopotential method implemented in CASTEP. The unit cell is marked by a parallelogram. Such choice avoids trajectories crossing or touching boundary of the unit cell. Top(blue atoms) and bottom(orange) layers of reactant(left, AA stacking) and product(right side, AB stacking) used in calculations. In order to avoid atoms crossing periodic boundary of a unit cell a slightly modified unit cell was introduced for AB stacked bilayer graphene(Fig 2).įigure 2. This choice of transformation is explained by a strong intralayer and weak interlayer interaction in bilayer graphene. One way to represent this reaction is to shift the whole top layer by one bond length. For AB stacked bilayer A subltattice of top layer is on top of B subltattice of the bottom layer and B sublattice of the top layer is hovering over the center of honeycombs of the bottom layer. (Source )For AA stacked bilayer the atoms are right on top of each other. Furthermore, the corrosive region of the Mg Pourbaix diagram can be suppressed if *OH adsorption is limited to certain low-energy active sites, where they form a stable hydroxide surface.Figure 1. This indicates that the presence of adsorbed *OH species provides an energetic driving force for water dissociation on Mg(0001). From this, it was shown that the enthalpy of hydroxylation of Mg(0001) becomes more negative with increasing surface coverage of *OH groups. Lastly, DFT-calculated reaction enthalpies were used to reproduce the bulk Mg Pourbaix diagram, and Pourbaix's formalism was extended to develop a theoretical Mg surface Pourbaix diagram. It was also found that the local structure of an adsorbed hydroxyl monolayer mimics that of the crystal structure of brucite, Mg(OH) 2. This is similar to previous first principles findings of oxygen adsorption on Mg(0001). Several electrochemical reactions were studied on the Mg surface, and it was found that dissociation of adsorbed water is thermodynamically favorable, and that the Mg(0001) surface has multiple ‘active sites’ that can accommodate adsorbed hydroxyl groups (*OH). The metal/water interface was modeled with a supercell approach, consisting of an extended metal surface coupled to an implicit solvent medium. Density functional theory (DFT) was used to study water dissociation on the Mg(0001) surface.


 0 kommentar(er)
0 kommentar(er)
